manganese electronic configuration|configuration of mn in mno2 : Clark Learn how to write the electron configuration of manganese (Mn) using orbit and orbital models. Compare the differences and similarities between the two methods and see examples and diagrams. Tingnan ang higit pa While NBI Clearance located at Duty Philippines opens at 8:00AM to 5:00PM. Duty Free Philippines only accepts NBI Application for Travel Abroad purposes only. Lastly, Robinsons Malls starts their NBI Clearance processing at 10:00AM to 7:00PM. All of the mentioned NBI Clearance Location is open Monday to Friday, except Holidays.
PH0 · valence electron of manganese
PH1 · mn+2 electron configuration
PH2 · iron electronic configuration
PH3 · electron configuration for every element
PH4 · electron configuration chart
PH5 · configuration of mn in mno2
PH6 · chromium electronic configuration
PH7 · chemistry electron configuration
PH8 · Iba pa
Create Yu-Gi-Oh! Cards. Designed specifically for the Yu-Gi-Oh! card game, TCG Editor allows you to create monsters, spells, traps and other types of cards with high quality output.
manganese electronic configuration*******Learn how to write the electron configuration of manganese (Mn) using orbit and orbital models. Compare the differences and similarities between the two methods and see examples and diagrams. Tingnan ang higit paThe total number of electrons in manganeseis twenty-five. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit pa
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit paAtoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground-state electron . Tingnan ang higit pa
1.1K. 155K views 4 years ago. To write the configuration for the Manganese ions, first we need to write the electron configuration for just Manganese (Mn). We first .
Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. . The atomic structure of Manganese includes four electron subshells. First subshell contains 2 electrons. Second subshell contains 8 electrons. Third subshell . So, the Manganese electron configuration can basically be written as the 1s22s22p63s23p64s23d5 for understanding purposes. The electron configuration is the significant chemical properties of the .Learn the electron configuration of manganese (Mn) and its main properties, such as oxidation states, applications and biological role. Find out how manganese is used .Electronic configuration of the Manganese atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5Electronic configuration of the Manganese atom in . Learn how to determine the electron configuration of different atoms based on their atomic numbers and quantum mechanics. Find out the oxidation states and .Manganese (Mn) has an atomic mass of 25. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.manganese electronic configurationLearn how to visualize the electron configuration of manganese (Mn) using an orbital diagram. See the distribution of electrons in the 1s, 2s, 2p, 3s, 3p, and 4s orbitals, .
Because of its valence electron configuration, it allows us to use it in different and unique ways that typically other elements cannot be used in. In biological systems manganese is a crucial component of vitamin \(B_1\). The pure metal is produced from its most common compound (\(MnO_2\)---10 th most abundant compound in the .
What is the electron configuration for manganese? View Solution. Q3. Element with the electronic configuration as [A r] 18 3 d 5 4 s 1 is placed in? View Solution. Q4. Calculate the effective nuclear charge of the last electron in an atom whose configuration is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 5. View Solution. Q5. Explanation: Mn has an atomic number of 25. Simply use this information to obtain its electronic configuration. Mn: 1s22s22p63s23p64s23d5 or simply [Ar]4s23d5 . Answer link. [Ar] 4s^2 3d^5 Mn has an atomic number of 25. Simply use this information to obtain its electronic configuration. Mn: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^5 or simply . Manganese, on the other hand, has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5 and a noble gas configuration of [Ar]4s 2 3d 5, resulting in one unpaired electron in each 3d sub-orbital. Similar to copper, the exchange energy is maximized since the 3d sub-orbital is exactly half-filled with 5 electrons without the need .
Electron configuration 3d 5 4s 2: Electrons per shell: 2, 8, 13, 2: . Manganese is a chemical element; it has symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s.Manganese (Mn) has an atomic mass of 25. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. ChemicalAid. . Electron Configuration [Ar] 4s 2 3d 5: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5: Orbital Diagram. Nuclear. Radioactive: No: Isotopes. Symbol Mass Number Relative Atomic Mass Isotopic .
In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is . For determining its electron configuration, we first lo. Today in this video, we will help you determine the electron configuration for the Manganese element.Mn (Manganese) is an element with position number 25 in the periodic table. Located in the IV period. Melting point: 1244 ℃. Density: 7.44 g/cm 3 . Electronic configuration of the Manganese atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5Electronic configuration of the Manganese atom in ascending order of the .La configuration électronique du manganèse est 1s2 2s2 2p6 3s2 3p6 3d5 4s2. Le manganèse est l'élément chimique du tableau périodique des éléments situé dans le groupe 7, son symbole est Mn et son numéro atomique est 25. On le trouve dans la nature sous forme d'élément libre, souvent combiné avec du fer et d'autres minéraux. The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2. The diagram below represents the electron configuration as an orbital diagram. Answer link. Refer to . The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .

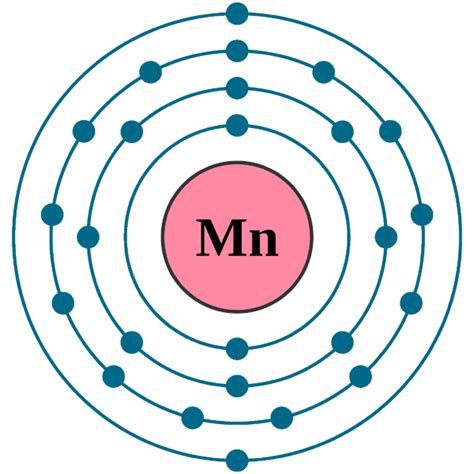
Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and . Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.configuration of mn in mno2 Mn: properties of free atoms. Manganese atoms have 25 electrons and the shell structure is 2.8.13.2. The ground state electron configuration of ground state gaseous neutral manganese is [ Ar ]. 3d5. 4s2 and the term symbol is 6S5/2. Schematic electronic configuration of manganese. The Kossel shell structure of manganese. The ground state electronic configuration of Mn2+ ion is 1s2 2s2 2p6 3s2 3p6 3d5. Mn 2+ ion is formed by the loss of two electrons from manganese (Mn) metal by ionization. Delta i H is the ionization enthalpy required to remove two electrons from manganese (Mn) metal, and its value is 1509 KJ/Mol.
manganese electronic configuration configuration of mn in mno2Manganese: Manganese is a d-block element having an atomic number 25 and atomic symbol Mn. It belongs to Group-7 and the fourth period. It is a hard silvery transition metal. Electronic configuration: The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration. Because of its valence electron configuration, it allows us to use it in different and unique ways that typically other elements cannot be used in. In biological systems manganese is a crucial component of vitamin \(B_1\). The pure metal is produced from its most common compound (\(MnO_2\)---10 th most abundant compound in the .
A PCB circuit board. One of the biggest challenges to creating a PCB project is finding the right components. Most designers find electronic components online, but that comes with its own set of unique problems. Components and footprints available online are not always easy to access and the resources themselves might not be reliable.
manganese electronic configuration|configuration of mn in mno2